How bats could help tomato farmers (and the U.S. Navy)
Bats, with their superb ability to echolocate, are inspiring advanced technologies — from better Navy sonar to gadgets that might deliver packages or help farmers manage crops. And engineers aren’t waiting for neuroscientists to work out every detail of how the bats’ brains manage the task.
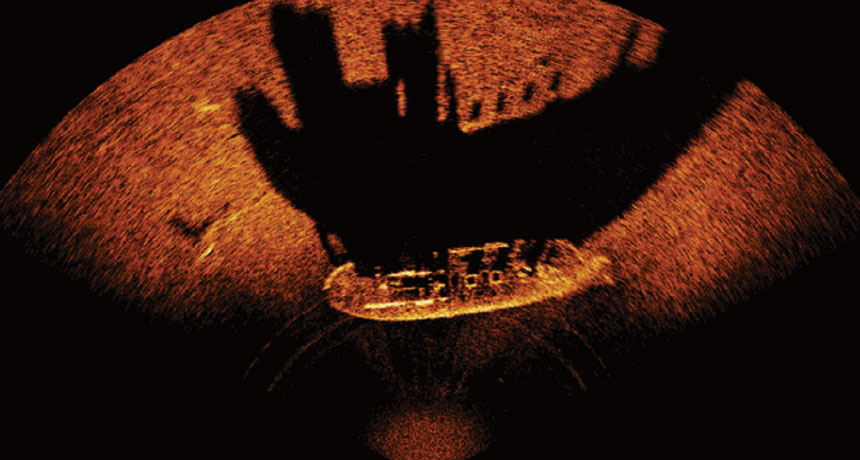
“We think we have enough information to be useful to us, to develop a bio-inspired sensor,” says research engineer Jason Gaudette of the Naval Undersea Warfare Center Newport Division in Rhode Island. Like bats, the Navy uses sonar to find and visualize objects in the deep. But current versions are far less elegant than the flying mammals’ system.
The Navy’s sonar arrays can be huge, encompassing hundreds of “ears” that listen for sonar pings from atop a submarine’s dome or trailing behind it in a long tail. Bats, Gaudette notes, dodge obstacles and find mosquito-sized meals with just two ears. He and colleagues have developed a bat-inspired prototype device that they hope can perform more like bats do. Mounted on the nose of a half-meter-long, torpedo-shaped autonomous undersea robot, the sonar system has one sound transmitter and three receivers (Gaudette hopes to eventually get that number down to two or even one).
The system uses algorithms inspired by research in bats to interpret returning sonar echoes for navigation. If it works, the system could help the Navy perform sonar imaging using less space and less money while offering sharper images, Gaudette says.
Researchers in Israel are hoping to help farmers with a bat-inspired kind of sonar. Neuroecologist Yossi Yovel of Tel Aviv University is creating computer algorithms describing how bats might interpret returning echoes to distinguish different plants.
Yovel collaborates with Avital Bechar, a researcher at the Institute of Agricultural Engineering near Rishon LeZion, Israel, who wants to help farmers predict their crops’ yield, which can vary widely from year to year. The same acre of tomato plants, for example, could yield 30 or 120 tons of fruit, Bechar estimates. Such a wide range puts farmers at a disadvantage in negotiating a price for crops and forces the farmers to guess how much equipment and how many pickers they’ll need at harvest time.
Bechar’s sonar system, which emits batlike sounds and records via microphones that mimic bat ears, can penetrate three rows of plants deep — farther than cameras could. Then it calculates the number of leaves and pounds of fruit per plant, based on Yovel’s algorithms. Bechar has mounted the scanner on a prototype robot and plans to affix it to a drone to count fruit in 15-meter-high date palms. The researchers also hope to add weed-detection capability. Bechar expects it to be “a game changer in agriculture, because it will reduce the unknowns.”
At Virginia Tech in Blacksburg, engineer Rolf Mueller is learning tricks from the physical structures of bats’ noses and ears. Certain bat groups, such as horseshoe bats (one species Mueller works with is Rhinolophus ferrumequinum), send out their echolocation calls through their noses, like a snort. Complex, fleshy formations called nose-leaves change the outgoing sound as it comes out of the nose. And the bats’ ears have more than 20 muscles, which rapidly change shape as the bat listens for echoes, Mueller says. That flexibility gives the animals more information, he suspects: “It’s like seeing the world with a different perspective, at the same time, [from] one echo.”
His group developed a prototype robot with mechanical “nose-leaves” and shape-shifting “ears,” and sent it zooming through forested areas on a zip line to record how the bot perceives trees and branches. Eventually, Mueller envisions an autonomous underwater bot or an airborne drone with a similar sonar setup. The drone could be useful for delivering packages in forested or otherwise complicated areas without crashing.